Abstract
Introduction: Ibrutinib is an orally administered Bruton's tyrosine kinase inhibitor (BTKi) approved in the US in 2014 for the treatment of patients with chronic lymphocytic leukemia (CLL) and CLL with 17p deletion. The goals of this study were to describe the patient population diagnosed with CLL and receiving treatment with ibrutinib in community practice, to evaluate ibrutinib discontinuation rates and reasons, and to further examine associated outcomes.
Methods: Adult pts with CLL who initiated ibrutinib between 1/Jan/ 2013 and 31/Oct/2015 were identified retrospectively fromelectronic health records (EHR) of McKesson Specialty Health and followed through 30/Apr/2016 or last visit date documented in the pts' medical chart. Pts in clinical trials or with another primary cancer diagnosis were excluded. Demographic, disease and treatment characteristics were abstracted from structured data elements of the EHR and supplemented by manual chart abstraction. Overall survival (OS) was analyzed using Kaplan Meier analysis.
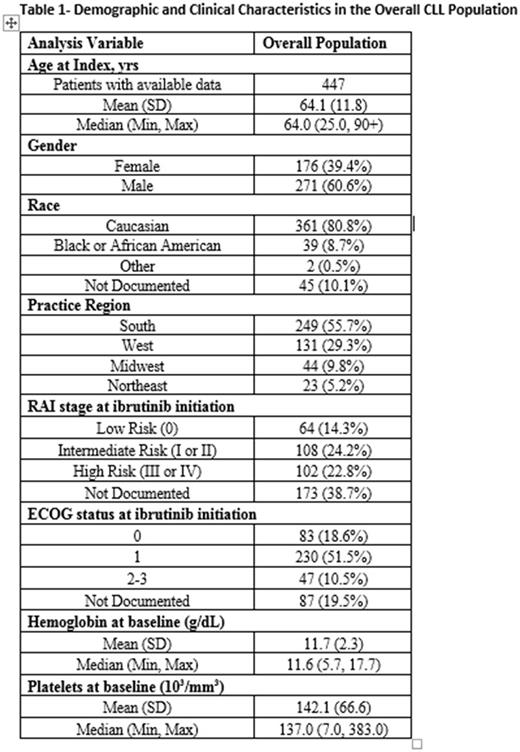
Results: 447 pts, with a median follow-up of 20.0 mos (range= 0.03-38.0), were identified for this study. At ibrutinib initiation, median age was 64.0 yrs; 60.4% were male, 47.0% were identified as intermediate or high risk CLL (RAI stages I through IV) and 70.0% had an ECOG performance status of 0 or 1 (Table 1). The median initial ibrutinib dose was 420 mg daily with 83% of subjects starting from this dose. Median duration of ibrutinib treatment was 15.2 mos (range=0.2-37.9).
Of 447 patients, 222 (49.7%) were continuing ibrutinib at the end of the observation period, 103 (23.0%) discontinued ibrutinib due to toxicity, 33 (7.4%) discontinued ibrutinib due to progression, 48 (10.7%) discontinued due to other reasons (adequate response achieved, financial, physician choice, patient choice) and 41 (9.2%) discontinued due to death.
Median OS from ibrutinib initiation was similar between subjects discontinuing due to toxicity and due to progression. Patients discontinuing ibrutinib due to toxicity had 12 and 24-month survival probabilities of 88.0% (95% CI 79.8, 93.0) and 70.5% (95% CI 57.5, 80.2) respectively; patients discontinuing ibrutinib due to progression had 12 and 24-month survival of 81.8% (95% CI 63.9, 91.4) and 75.3% (95% CI 56.5, 86.8) respectively. Patients who continued ibrutinib had a 99.5% (95% CI 96.8, 99.9) overall survival at 24 months.
Conclusions: This real world study evaluated ibrutinib treatment patterns and identified reasons for discontinuation and the subsequent outcomes in patients diagnosed with CLL. New treatment options, such as novel BTK inhibitors with improved tolerability, are needed to reduce the rate of discontinuation due to toxicities and thus extend the clinical benefits of continuous BTK inhibition in this disease setting.
Sharman: Gilead Sciences, Inc.: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Genentech: Research Funding. Black-Shinn: McKesson Specialty Health: Employment. Clark: McKesson Specialty Health: Employment, Equity Ownership. Bitman: Acerta Pharma: Employment; Astra Zeneca: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal